Introduction
Zirconia, or zirconium dioxide (ZrO2), has become a prominent material in modern dentistry due to its exceptional properties. Known for its high strength, biocompatibility, and aesthetic appeal, zirconia is widely used in various dental applications, revolutionizing restorative and cosmetic dentistry.
Properties of Zirconia
- High Strength: Zirconia is renowned for its high flexural strength and fracture toughness, making it an ideal material for dental restorations that require durability and resistance to chewing forces.
- Biocompatibility: Zirconia is highly biocompatible, meaning it is well-tolerated by the body. This property reduces the risk of allergic reactions and ensures long-term success of dental implants and restorations.
- Aesthetic Appeal: With its tooth-like color and translucency, zirconia offers excellent aesthetic results. It can be shaded to match natural teeth, making it suitable for visible restorations.
- Corrosion and Wear Resistance: Zirconia is resistant to corrosion and wear, ensuring longevity and maintaining its integrity over time, even in the harsh oral environment.
- Low Thermal Conductivity: Zirconia’s low thermal conductivity protects the pulp of the tooth from temperature changes, enhancing patient comfort.
Applications in Dentistry
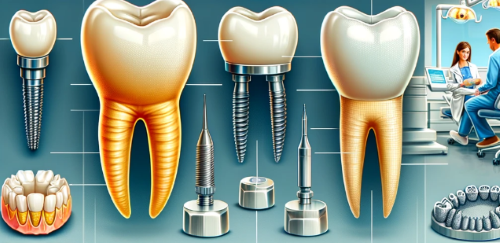
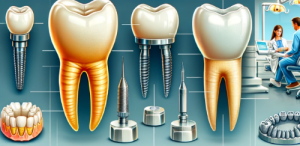
- Crowns and Bridges: Zirconia is extensively used for fabricating dental crowns and bridges due to its strength and natural appearance. It can be used for both anterior and posterior restorations, providing durability and aesthetics.
- Dental Implants: Zirconia implants are an alternative to traditional titanium implants. They offer excellent osseointegration, biocompatibility, and a metal-free option for patients with metal sensitivities.
- Inlays and Onlays: Zirconia is used for inlays and onlays, providing a strong and durable solution for restoring decayed or damaged teeth while preserving more of the natural tooth structure.
- Orthodontic Brackets: Zirconia is used in orthodontic brackets due to its strength and tooth-colored appearance, providing a more aesthetically pleasing alternative to metal brackets.
- Veneers: Zirconia veneers are used to improve the appearance of teeth. They are strong, durable, and can be matched to the color of natural teeth, providing a seamless look.
- Full Mouth Reconstructions: For patients requiring extensive dental work, zirconia is used in full mouth reconstructions, providing a durable and aesthetically pleasing solution for multiple restorations.
Advantages of Zirconia in Dentistry
- Durability: Zirconia’s high strength and resistance to fracture and wear make it one of the most durable materials available for dental restorations.
- Aesthetics: Its ability to mimic the natural color and translucency of teeth makes zirconia an excellent choice for visible restorations, enhancing the patient’s smile.
- Biocompatibility: Zirconia’s biocompatibility ensures that it integrates well with the body, reducing the risk of adverse reactions and promoting long-term success.
- Minimal Preparation: Zirconia restorations often require minimal tooth preparation, preserving more of the natural tooth structure.
- Versatility: Zirconia can be used in a wide range of dental applications, from single crowns to full mouth reconstructions, making it a versatile material for various dental needs.
Future Trends and Developments
- Improved Aesthetics: Ongoing research is focused on enhancing the aesthetic properties of zirconia, such as improving translucency and color matching, to make it even more indistinguishable from natural teeth.
- Enhanced Osseointegration: Developments in surface treatments and coatings aim to improve the osseointegration of zirconia implants, enhancing their stability and success rates.
- Digital Dentistry: The integration of CAD/CAM technology with zirconia restorations allows for precise, customized restorations with improved fit and function, reducing the time required for dental procedures.
- Bioactive Zirconia: Research is exploring the development of bioactive zirconia that can promote bone growth and integration, further enhancing its suitability for dental implants and other applications.
Conclusion
Zirconia has significantly impacted modern dentistry, offering a combination of strength, biocompatibility, and aesthetic appeal. Its versatility makes it suitable for a wide range of dental applications, from crowns and bridges to implants and veneers.
As technology advances and research continues, the use of zirconia in dentistry is likely to expand, offering even more innovative and effective solutions for dental care. For more information, please check Advanced Refractory Metals (ARM).