Introduction
Zirconium sponge is a highly purified form of zirconium that plays a pivotal role in several advanced industries. Its unique properties, including exceptional corrosion resistance, high melting point, and mechanical stability, make it indispensable in aerospace and chemical processing applications. This article discusses the significance of zirconium sponge in these sectors, exploring its properties, production, and key uses.

Properties of Zirconium Sponge
1.Corrosion Resistance: Zirconium sponge exhibits outstanding resistance to corrosion, particularly in harsh chemical environments. This property is crucial for applications in chemical processing where equipment is exposed to corrosive substances.
- High Melting Point: With a melting point of 1855°C, zirconium sponge can withstand extreme temperatures, making it suitable for high-temperature aerospace applications.
- Mechanical Stability: The material’s excellent mechanical properties ensure durability and reliability, essential for components subjected to high stress and wear.
- Low Neutron-Capture Cross-Section: This property is particularly valuable in the nuclear industry, but it also benefits aerospace applications where materials must endure radiation.
Production of Zirconium Sponge
The production of zirconium sponge primarily involves the Kroll process, which includes the following steps:
- Chlorination: Zirconium ore (zircon) is processed to produce zirconium tetrachloride (ZrCl4).
- Reduction: Zirconium tetrachloride is reduced with magnesium in a high-temperature reactor, producing zirconium sponge and magnesium chloride as a byproduct.
- Purification: The sponge zirconium is then purified to remove any residual magnesium and other impurities, resulting in high-purity zirconium suitable for industrial applications.
Applications in the Aerospace Industry
- High-Temperature Components: The aerospace industry demands materials that can withstand extreme temperatures and maintain structural integrity. Zirconium sponge is used in the production of components such as turbine blades, engine parts, and thermal barrier coatings.
- Alloy Production: Zirconium is alloyed with other metals to enhance their properties, producing materials that offer a combination of light weight, strength, and resistance to thermal and mechanical stress.
- Protective Coatings: Due to its corrosion resistance, zirconium sponge is used to manufacture protective coatings for various aerospace components, extending their lifespan and reliability.
Applications in the Chemical Industry
- Chemical Processing Equipment: The chemical industry benefits from zirconium sponge’s exceptional resistance to corrosion. It is used to fabricate reactors, pipes, valves, and heat exchangers that handle aggressive chemicals and high temperatures.
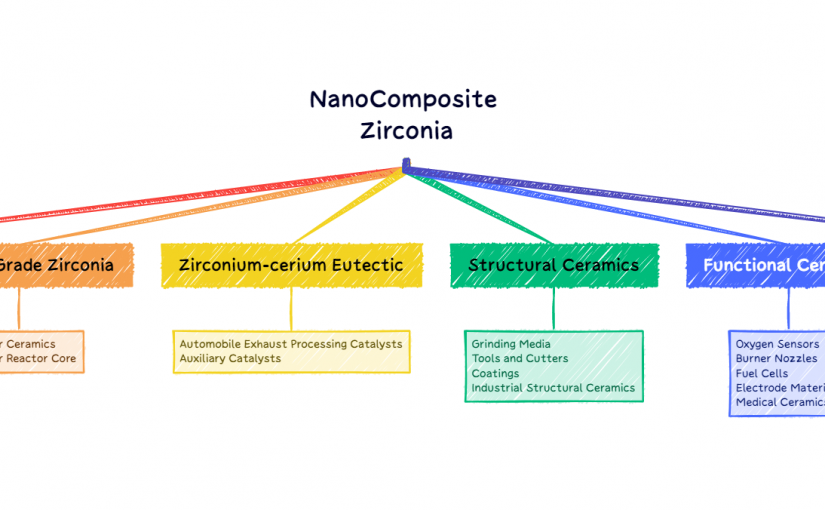
- Catalysts and Catalyst Supports: Zirconium compounds are used as catalysts and catalyst supports in chemical reactions, improving efficiency and selectivity in processes such as hydrocarbon cracking and polymerization.
- Storage and Transport Containers: Containers and vessels made from zirconium sponge are used to store and transport corrosive chemicals safely, reducing the risk of leaks and contamination.
Future Prospects and Innovations
The demand for zirconium sponge is expected to grow as industries continue to seek materials that offer superior performance under extreme conditions. Innovations in production techniques and new applications are likely to enhance its role in the aerospace and chemical sectors. Research is ongoing to develop zirconium-based materials with even better properties, potentially opening up new uses in emerging technologies such as space exploration and advanced manufacturing.
Conclusion
Zirconium sponge is a critical material for the aerospace and chemical industries, offering unparalleled properties that meet the rigorous demands of these sectors. Its exceptional corrosion resistance, high melting point, and mechanical stability make it indispensable for high-performance applications. As technology advances and industrial requirements evolve, zirconium sponge will continue to be a material of choice, driving innovation and efficiency in aerospace and chemical processing. For more information, please check Advanced Refractory Metals (ARM).