Vacuum distillation refers to the process of removing metal magnesium and MgCl2 in sponge zirconium by distillation under the condition of lower pressure than normal pressure. The zirconium sponge produced by distillation is then vacuum cast into metal or alloy, which is used in industrial sectors such as atomic energy, metallurgy, and chemistry.
Principle of Vacuum Distillation
The raw material of vacuum distillation is generally the product of the reduction of zirconium tetrachloride and magnesium, containing 55% to 60% of zirconium, 25% to 30% of magnesium, 10% to 15% of MgCl2 and a small amount of Zrcl3 and ZrCl2.
The vapor pressures of these components are different at a certain temperature and pressure. For example, in the standard state, the boiling point of magnesium is 1380K, MgCl2 is 1691K, and zirconium is 4673K; at normal pressure and 1173K temperature, the equilibrium vapor pressure of magnesium is 13332.2Pa, MgCl2 is 999.9Pa, and zirconium is less than 130μPa. Therefore, by controlling the appropriate distillation temperature and pressure, zirconium and other components can be separated.
In addition, under a 10Pa vacuum, the boiling points of magnesium and magnesium chloride dropped to 789K and 950K, respectively, and the volatilization rate was many times greater than that of atmospheric distillation. Therefore, the use of vacuum distillation can shorten the distillation time, reduce the distillation temperature, improve the separation effect, and avoid the formation of Zr–Fe alloys that contaminate the zirconium sponge and iron.
System for vacuum distillation
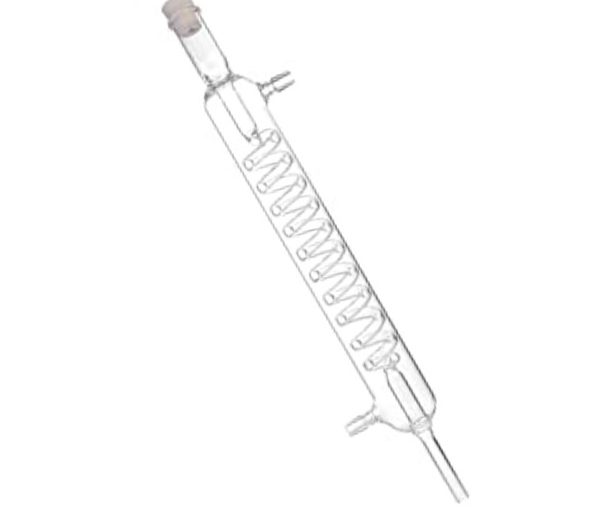
The vacuum distillation system is mainly composed of a distillation furnace, a distillation tank, a condensation sleeve, a condenser, a heat shield, and a vacuum system.
According to the installation position of the condenser, it can be divided into two types: upper cooling type and lower cooling type, and the structure of the two is basically the same. In industrial production, the distillation furnace and the reduction reactor are the same. Therefore, the structural design and material selection of the reduction reactor should take into account the requirements of the reduction and distillation processes.
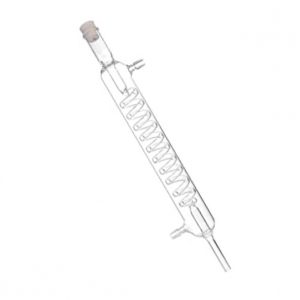
The condenser is a hoistable bell-shaped cylindrical tank with a cooling water jacket. The condensing sleeve is cylindrical, and the condensing area is set according to the amount of condensate discharged by distillation. A heat shield is arranged between the distillation tank and the condenser, the function of which is to reduce the radiant heat from the heating area to the condenser, without hindering the passage of the airflow escaping from the distillation tank. In order to improve the thermal insulation effect, most of the heat shields are multi-layer structures.
The structure of the distillation furnace is the same as that of the reduction reactor, but the furnace shell of the former should be sealed and connected to a vacuum device. During operation, the furnace is in a low vacuum state to reduce the external pressure on the distillation tank and prevent the latter from deforming.
Process of vacuum distillation
- The reducing crucible together with the reaction product is placed upside down in the distillation pot of the distillation furnace.
- The distillation tank was evacuated to 13.3-1mPa, and then the distillation furnace was heated to a temperature of 573-673K and kept at a constant temperature for 1-4 hours to remove the crystal water adsorbed by MgCl2 during the assembly process of the distillation equipment.
- Then the furnace temperature was raised to 1023-1073K. At this time, since the metal magnesium and MgCl2 begin to volatilize in large quantities, the vacuum degree drops sharply, and the heating rate needs to be controlled well.
- After the distillation enters the constant temperature stage, the temperature should be controlled at 1223-1273K.
- After 20-25 hours of constant temperature, when the vacuum in the distillation tank rises to less than 1Pa and tends to be stable for a certain period of time, the residual amount of volatiles is very small, and the distillation operation is over.
In the whole distillation process, process parameters such as distillation temperature, vacuum degree and distillation time should be well controlled. It takes about 50 to 60 hours to distill 700 to 800 kg of zirconium. The zirconium sponge produced by distillation is self-igniting. When the distillation tank is cooled to 323K temperature, a mixed gas consisting of 60% dry air and 40% indoor air should be slowly introduced to reduce the surface activity of the sponge zirconium and make it passivated before it is released.
Product handling after vacuum distillation
Zirconium sponge is a hard and tough metal. Usually, the zirconium lump is cut into pieces by a vertical hydraulic press equipped with a cutter, and then it is medium and finely crushed to make the particle size reach 5-25mm, and then sieved, classified, mixed in batches, and packed with argon. The typical impurity content (mass fraction ω/%) of sponge zirconium is Fe 0.08, Al 0.006, Mg 0.002-0.02, Cl 0.001-0.04, O 0.08-0.1, N 0.002-0.004.
Sponge zirconium taken out from the reduction crucible can be divided into four types: A, B, C, and D.
- A type of zirconium sponge accounts for about 35% of the total and is a bulk dense metal that contains almost no metal magnesium and MgCl2.
- B-type zirconium sponge accounts for about 20% of the total. It is the product formed in the initial stage of the reaction, which is plate-shaped and about 10mm thick. In addition to metal magnesium, it contains a lot of iron and nitrogen impurities.
- C-type sponge zirconium accounts for about 35% of the total, is a light and porous sponge with the least impurities but contains a considerable amount of metal magnesium and chloride in the pores.
- D-type zirconium sponge is a product that is close to the crucible wall and contains a lot of impurities. Generally, it is returned to the chlorination treatment to recover the zirconium in it.
For more information, please visit https://www.samaterials.com/70-zirconium.html.