Introduction
Coatings and surface treatments for zirconium alloys are essential for enhancing their corrosion resistance, wear resistance, and biocompatibility. They can also improve performance characteristics for specific applications. Besides, zirconium alloys benefit from additional surface treatments to extend their lifespan and functionality, especially in more aggressive environments or when additional properties are needed.
This article explores various coatings and surface treatments designed for zirconium alloys. Hope that you can learn about their applications and benefits.
1. Anodizing for Zirconium Alloys
Anodizing is a process that electrochemically alters a metal surface to produce a decorative, robust, and corrosion-resistant anodic oxide layer, effectively enhancing the metal’s natural oxide surface layer.
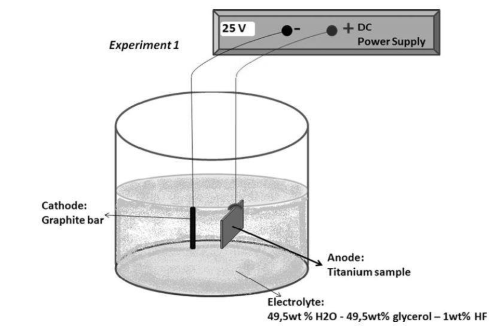
These metals have become a popular choice for both protective and aesthetic purposes in various industries, such as automotive, aerospace, consumer electronics, and construction.
1. Thermal Spraying
Thermal spraying is a coating process where melted (or heated) materials are sprayed onto a surface. The coating material, in the form of powder or wire, is heated to a molten or semi-molten state and accelerated towards the target substrate. Thermal spraying creates a strong bond to the surface upon cooling.
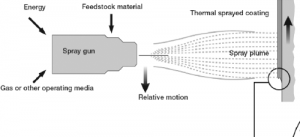
Each is suitable for different materials and applications ranging from aerospace and automotive to biomedical and electronics.
1. Chemical Vapor Deposition (CVD) and Physical Vapor Deposition (PVD)
Chemical Vapor Deposition (CVD) and Physical Vapor Deposition (PVD) are two advanced techniques for applying thin film coatings onto various substrates.
- CVD:
CVD involves chemical reactions between gaseous precursors and the substrate surface. It results in the deposition of a solid material. This method is excellent for coatings that require high purity and uniform thickness, even on complex geometries. It’s useful for semiconductor devices, corrosion-resistant coatings, and high-performance tool coatings.
- PVD:
PVD, on the other hand, physically transfers material from a source to the substrate in a vacuum environment. Techniques under PVD include sputtering and evaporation, allowing for the deposition of metals, alloys, and ceramic coatings. PVD coatings are notable for their high density, excellent adhesion, and uniformity, and they are ideal for aerospace components, medical implants, and cutting tools.
2. Electroplating
Electroplating can apply a metal coating, such as nickel or gold, onto zirconium alloys. This process can improve the alloy’s appearance, corrosion resistance, and electrical conductivity. Electroplating is often used in electronic components, decorative items, and applications requiring enhanced conductivity.
3. Passivation
Passivation involves treating the zirconium alloy with a chemical solution, usually an acid, to remove surface contaminants and enhance the naturally occurring oxide layer’s protective qualities. This process improves corrosion resistance by making the surface more passive and less likely to react with its environment. Passivation’s common uses are in the chemical processing industry and in medical device manufacturing.
4. Sol-Gel Coatings
The sol-gel process can create thin, uniform ceramic coatings on zirconium alloys. These coatings can provide excellent chemical stability, corrosion resistance, and thermal protection. Sol-gel coatings are versatile and can be tailored to include various functional materials for specific applications, including optics, electronics, and biomedical devices.
The following table succinctly captures the essence, features, and typical applications of each method. You can check this clear guide to select the appropriate coating process for various requirements.
| Methods | Definition | Features | Applications |
| Anodizing | An electrochemical process enhancing the natural oxide layer for a decorative, durable finish. | Increases corrosion and wear resistance; accepts vibrant colors. | Automotive, aerospace, consumer electronics, and construction. |
| Thermal Spraying | Coating process spraying melted materials onto a surface. | Provides protection against wear, corrosion, and heat; restores/increases dimensions. | Aerospace, automotive, biomedical, and electronics. |
| Chemical Vapor Deposition (CVD) | Chemical reactions between gaseous precursors and the substrate, depositing a solid material. | High purity, uniform thickness, suitable for complex geometries. | Semiconductor devices, corrosion-resistant coatings, and tool coatings. |
| Physical Vapor Deposition (PVD) | Transfers material from a source to the substrate in a vacuum. | High density, excellent adhesion, and uniformity. | Aerospace components, medical implants, and cutting tools. |
| Electroplating | Applies a metal coating, like nickel or gold, onto substrates. | Improves appearance, corrosion resistance, and electrical conductivity. | Electronic components, decorative items, and enhanced conductivity applications. |
| Passivation | Treating the alloy with a chemical solution to remove contaminants and enhance oxide layer protection. | Improves corrosion resistance; makes the surface less reactive. | Chemical processing industry and medical device manufacturing. |
| Sol-Gel Coatings | Creates thin, uniform ceramic coatings. | Offers chemical stability, corrosion resistance, and thermal protection. | Optics, electronics, and biomedical devices. |
Conclusion
Coatings and surface treatments for zirconium alloys are critical for enhancing their properties and expanding their application range. By selecting appropriate treatments, it’s possible to significantly improve the performance of zirconium alloys in various industrial, medical, and technological applications.
As technology advances, new coating techniques and materials will likely emerge, further enhancing the capabilities of zirconium alloys. For more information related to zirconium alloys, please check Advanced Refractory Metals (ARM).
Reference:
[1] Mihajlović, Dragana & Cvijović-Alagić, Ivana & Dimic, Ivana & Djokic, Veljko & Rakin, Marko. (2016). Anodization of Ti-based materials for biomedical applications: A review. Metallurgical and Materials Engineering. 22. 129-143. 10.30544/209.
[2] Ahmad, Zaki & Khan, Asad & Farooq, Robina & Saif, Tahir & Mastoi, Naila. (2016). Mechanism of Corrosion and Erosion Resistance of Plasma‐ Sprayed Nanostructured Coatings. 10.5772/64316.